According to wave mechanical concept " The maximum probability of the existence of the electron is at a given distance from the nucleus " . According to Bohr's calculation and according to wave mechanics the value of that distance have same value.
Wave mechanics says that "The space around the nucleus where probability of finding of electron is very high " is called atomic orbitals.
1. Shape of S- orbitals--
--For S-orbitals l = 0 therefore m = 0 but the value of n can vary.
--All the s orbitals has spherical shape , maximum electron find in this region.
-- S orbitals can hold maximum 2 electrons, each with opposite spin.

-There are total 5 possible values for orbitals
-Above picture shows that first four d-orbital are double dumbell shape while last dz orbital is dumble shape with a collar in xy plane.It means maximum electron can be find in that given orientation or in that planes.
-For d orbitals n = 3 onwards.
maximum numbers of electron in d- orbitals = 10 .
4. Shape of f- orbitals-
- Shape of f orbitals are very complex then d- orbitals ..
-There are total 7 f- orbitals .
-Maximum number of electron found in f - orbitals = 14.
Filling of electron in s, p, d, f orbitals follow the given order--
1s->2s->2p-> 3s-> 3p-> 4s-> 3d-> 4p.....so on
Energy of orbitals in increase in following order--
1s< 2s<2p< 3s< 3p< 4s <3d< 4p ...

=> Value of energy and radius of orbitals increases with increase in number of orbitals or with increase in Principal quantum number n (shell).l
Wave mechanics says that "The space around the nucleus where probability of finding of electron is very high " is called atomic orbitals.
1. Shape of S- orbitals--
--For S-orbitals l = 0 therefore m = 0 but the value of n can vary.
--All the s orbitals has spherical shape , maximum electron find in this region.
-- S orbitals can hold maximum 2 electrons, each with opposite spin.
2. Shape of P- orbitals -
- All orbitals have l (Azimuthal q. number) = 1 and 3 possible values of m (-1 , 0, +1)
-All the p orbitals (Px, Py, Pz) are dumbell shape ,the region where the probability of finding of electron is very high.

3. Shapes of d- orbitals-
-There are total 5 possible values for orbitals
dz2,
|
dxz
|
,dyz
|
, dxy
|
, dx2−y2
|
-Above picture shows that first four d-orbital are double dumbell shape while last dz orbital is dumble shape with a collar in xy plane.It means maximum electron can be find in that given orientation or in that planes.
-For d orbitals n = 3 onwards.
maximum numbers of electron in d- orbitals = 10 .
4. Shape of f- orbitals-
- Shape of f orbitals are very complex then d- orbitals ..
-There are total 7 f- orbitals .
-Maximum number of electron found in f - orbitals = 14.
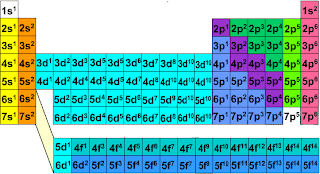
Place of s, p, d, f orbitals in periodic table--
Filling of electron in s, p, d, f orbitals follow the given order--
1s->2s->2p-> 3s-> 3p-> 4s-> 3d-> 4p.....so on
Energy of orbitals in increase in following order--
1s< 2s<2p< 3s< 3p< 4s <3d< 4p ...

=> Value of energy and radius of orbitals increases with increase in number of orbitals or with increase in Principal quantum number n (shell).l